1. The 24-hour mean of PM 2.5 should not exceed 15 ug/m³ and annual mean of PM 2.5 should not exceed 5 µg/m³.
2. In a year, the highest levels of ozone pollution occur during the periods of inclement weather.
3. PM-10 can penetrate the lung barrier and enter the bloodstream.
4. Excessive ozone in the air can trigger asthma.
Which of the statements given above are correct?
(a) 1, 3 and 4
(b) 1 and 4 only
(c) 2, 3 and 4
(d) 1 and 2 only
6. Ans: b
Explanation:
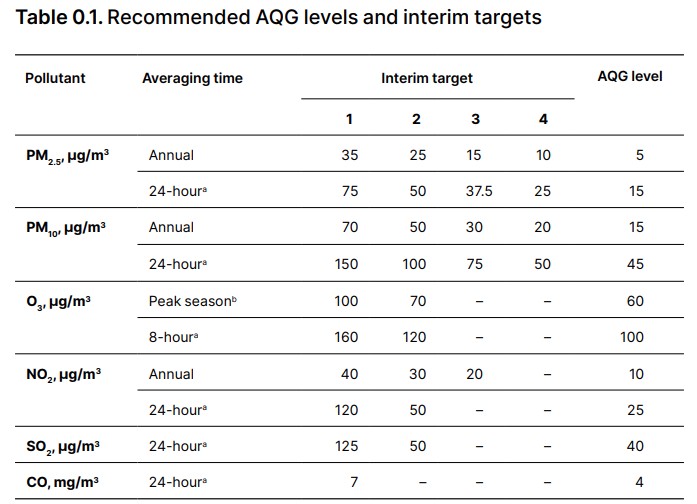
Hence, statement 1 is correct.
In the stratosphere, ozone molecules play an essential role – absorbing ultraviolet radiation from the Sun and shielding Earth from dangerous rays. But in the troposphere, near ground level, ozone molecules are air pollutants.
A small amount of ozone does occur naturally at ground level. Plants and soil release some. Some migrates down from the stratosphere. Most of the ozone that is found near the ground comes from vehicle exhaust and emissions from factories, power plants, and refineries.
Unlike most other air pollutants, ozone as apollutant is not directly emitted into the air. Tropospheric ozone is formed by the interaction of sunlight, particularly ultraviolet light, with hydrocarbons and nitrogen oxides, which are emitted by automobile tailpipes and smokestacks. In urban areas, high ozone levels usually occur during warm summer months.
Typically, ozone levels reach their peak in mid to late afternoon, after exhaust fumes from morning rush hour have had time to react in sunlight. A hot, sunny, still day is the perfect environment for the production of ozone pollution. At the end of the day, as the Sun starts to set, the production of ozone begins to subside. To form, ozone needs sunshine to fuel the chemical reaction. Though ozone is present in winter as well, the levels are lower owing to low temperatures, as it needs sharp sun to aid its formation.
Good ozone, bad ozone
Scientists have divided the atmosphere into different layers, each with a name. The layer closest to the ground, where we live and fly in jets, is called the troposphere [TRO-po-sphere]. Above that layer is the stratosphere [STRAT-o-sphere], which goes to about 30 miles high.
Ultraviolet radiation from the Sun causes sunburns and skin cancer. Ozone high in the stratosphere shields us from much of this ultraviolet radiation.- That’s good.
But at the top of the troposphere, ozone acts as a greenhouse gas and adds to global warming. That’s bad.
In the middle region of the troposphere, ozone helps to clean the atmosphere of certain pollutants.- That’s good.
But in the atmosphere close to Earth’s surface where we live, ozone adds to smog and is hard on plants and animals, including us. That’s bad.
Hence, statement 2 is incorrect.
Ozone pollution induces respiratory problems. When it’s inhaled, ozone can damage lung tissues. Ozone is harmful to all types of cells. It can impair an athlete’s performance, create more frequent attacks for individuals with asthma, cause eye irritation, chest pain, coughing, nausea, headaches and chest congestion. It can worsen heart disease, bronchitis, and emphysema.
Ozone also damages materials like rubber, textile dyes, fibers, and certain paints. These materials can be weakened or degraded by exposure to ozone. Some elastic materials can become brittle and crack, while paints and fabric dyes may fade more quickly.
Hence, statement 4 is correct.
Owing to its reactive nature, Ozone has 1-hour and 8-hour standards compared to particulate matter (PM) which has a 24-hour standard, because even a short-term exposure to the gas can worsen respiratory conditions. These standards are prescribed by the CPCB among one of the twelve (12) pollutants under National Ambient Air Quality Standard (NAAQS), 2009. The ambient air quality standards for Ozone (O3) is prescribed as 100 µg/m3 for 8-hourly monitored value and 180 µg/m3 for 1-hourly monitored value for industrial, residential, rural and ecological sensitive area. As per the NAAQS, the method of measurement of Ozone (O3) in ambient air is UV photometric, Chemiluminescence, and Chemical method.
Particulate matter:
PM stands for particulate matter (also called particle pollution): the term for a mixture of solid particles and liquid droplets found in the air. Some particles, such as dust, dirt, soot, or smoke, are large or dark enough to be seen with the naked eye. Others are so small they can only be detected using an electron microscope.
It has been found that PMs with an aerodynamic diameter smaller than 10 µm have a greater impact on human health. One group of PM identified, PM2.5, have small diameters, however large surface areas and may therefore be capable of carrying various toxic stuffs, passing through the filtration of nose hair, reaching the end of the respiratory tract with airflow and accumulate there by diffusion, damaging other parts of the body through air exchange in the lungs and then entering in the blood stream. PM2.5 causes asthma, respiratory inflammation, jeopardizes lung functions and even promotes cancers and impact on human respiratory system.
Hence, statement 3 is incorrect.
Read: Previous Year UPSC Environment Questions (PYQs) With Explanation 2022


